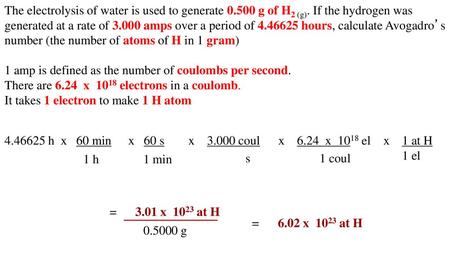
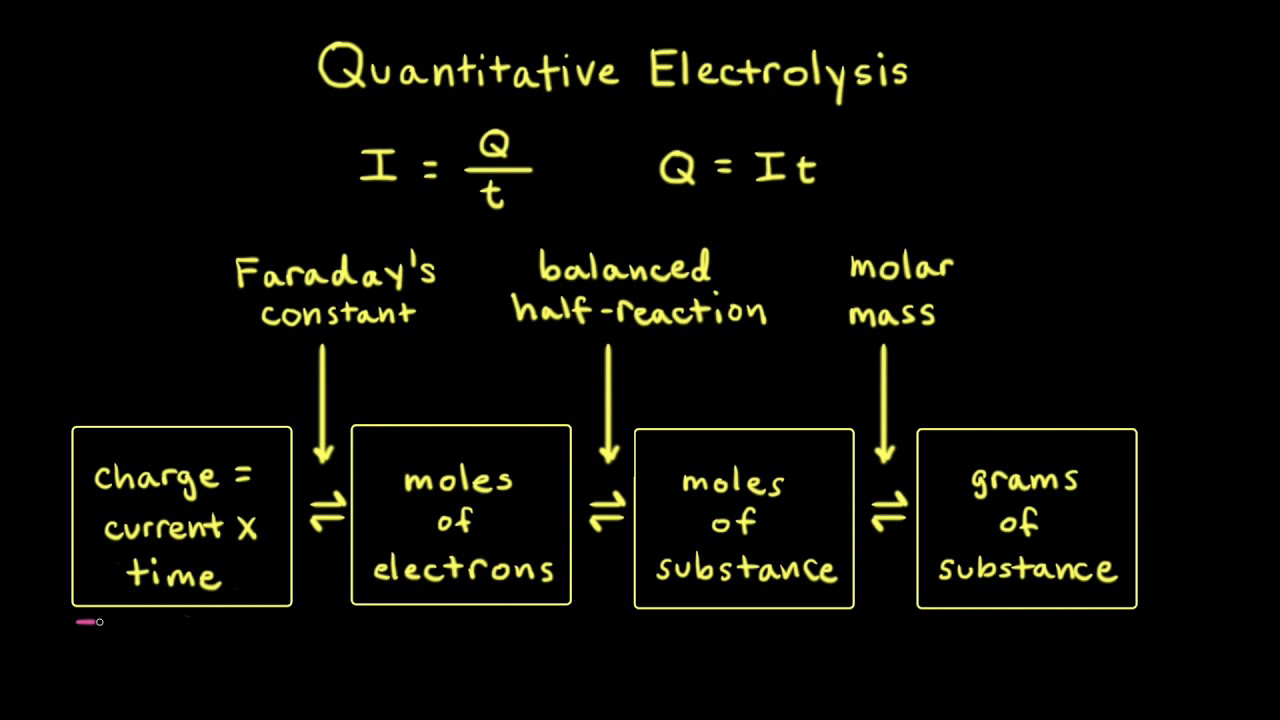
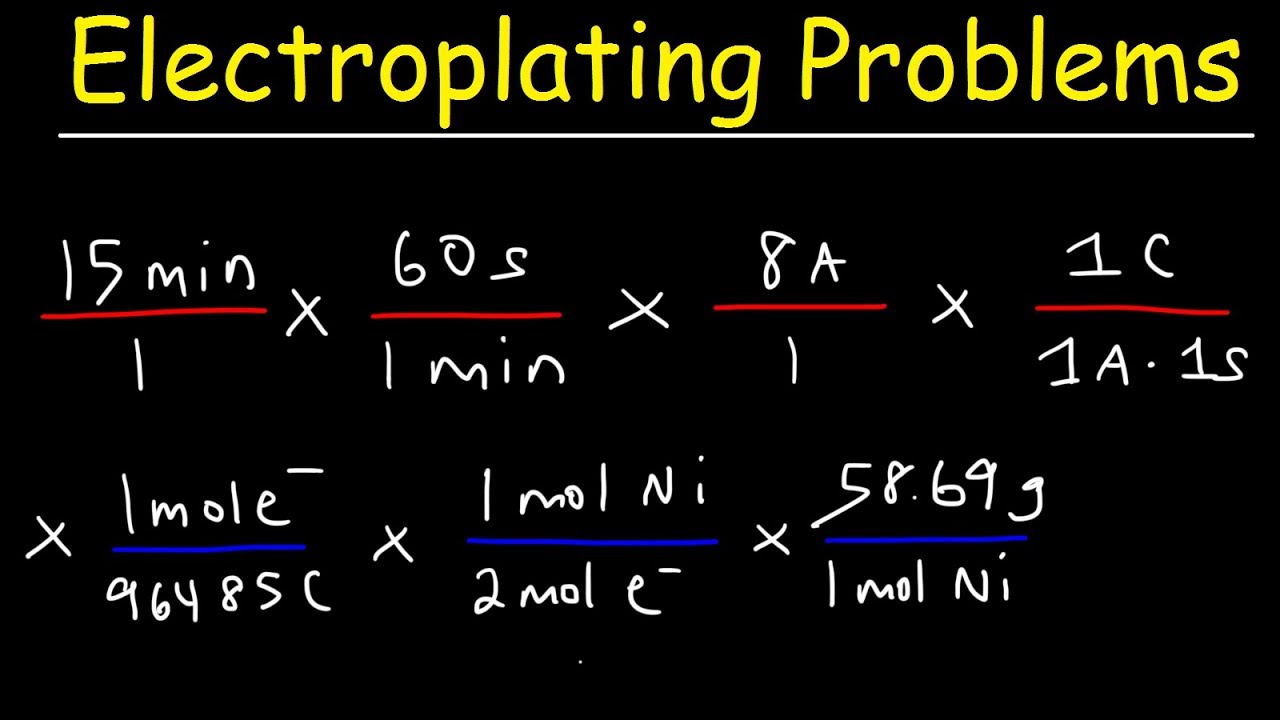
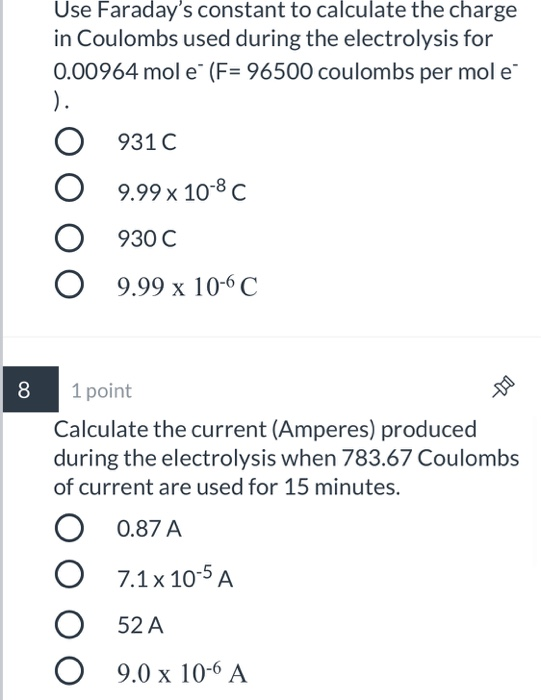
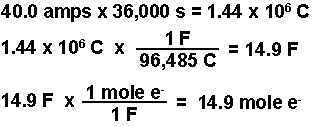During the electrolysis, a current of 0.72A was passed through the electrolyte for 15 munites.Calculate the volume of gas produced at the anode.(1 Faraday = 96...
Welcome to Chem Zipper.com......: During electro refining of Cu how much time is needed to produce 250g Cu on the cathode if the current is kept at 11 A?

SOLVED: Electrolysis of aqueous solution of 0.1 M CuBrz was carried out using inert electrode at 25 Determine the products of electrolysis at anode and cathode: Justify. Calculate the mass of product