The aqueous solution of transition metal salt changes colour from pink to blue, when concentrated hydrochloric acid is added to it. The change in colour is due to

Complete a net ionic equation for each proton-transfer reaction using curved arrows to show the flow of electron pairs. Write Lewis structures for all starting materials and products, label the original acid

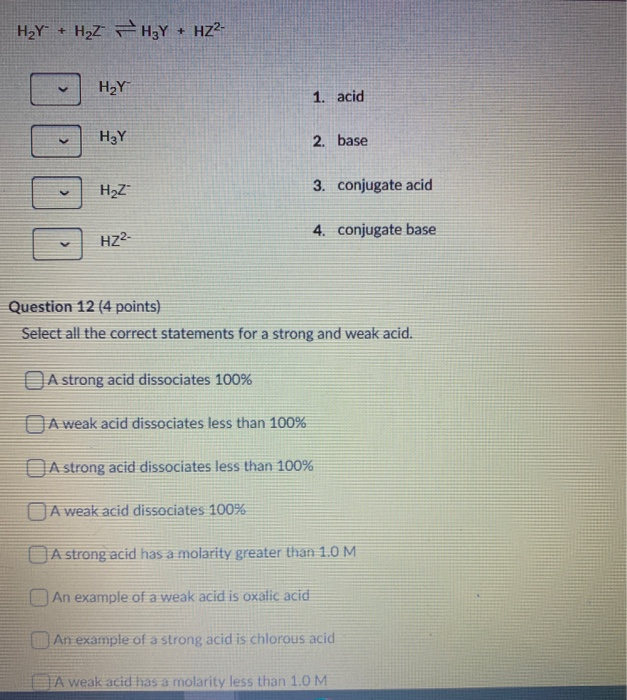
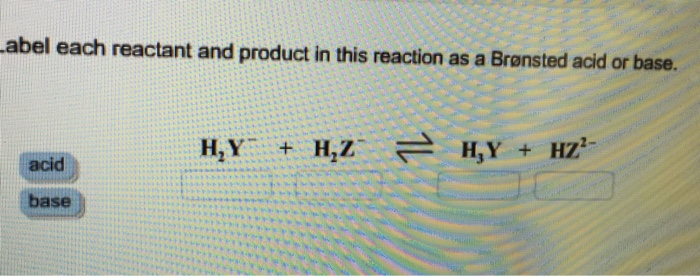
SOLVED: Identify each reactant and product in this reaction as a Bronsted acid or base. Hz Y- + HzZ acid base H; Y + HZ2 - base acid Answer Bank base acid

Solved: Identify each reactant and product in this reaction as a Brønsted acid or base.H,Y +H,Z - Brainly.com

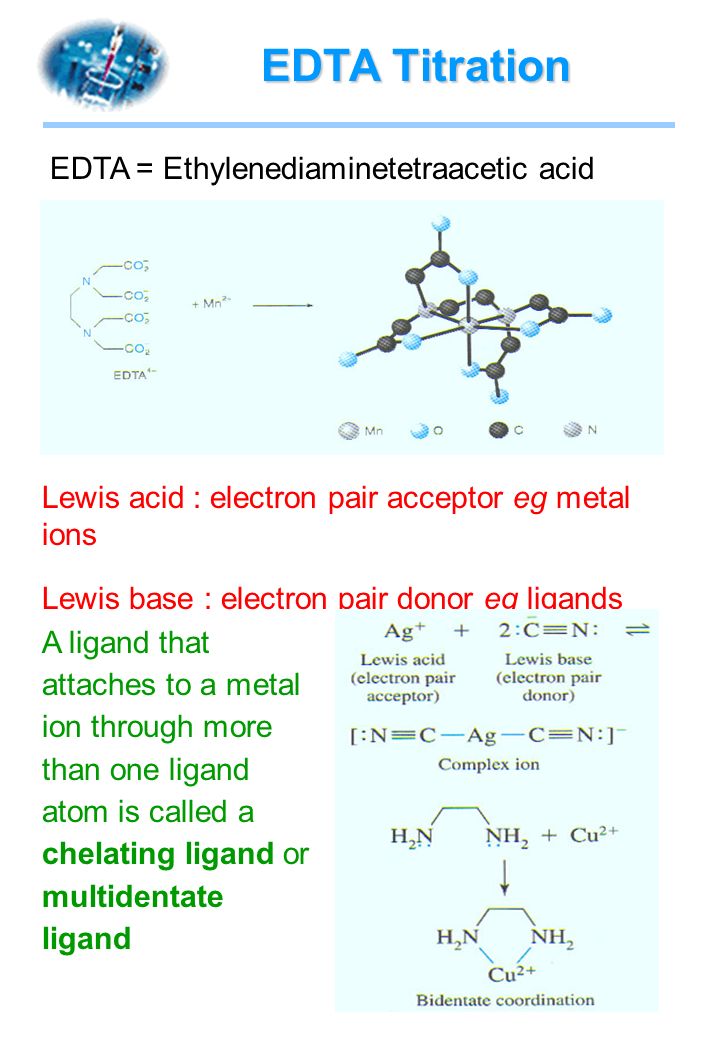
Complete the equation for the reaction between the following Lewis acid-base pair. Label which starting material is the Lewis acid and which is the Lewis base, and use curved arrows to show
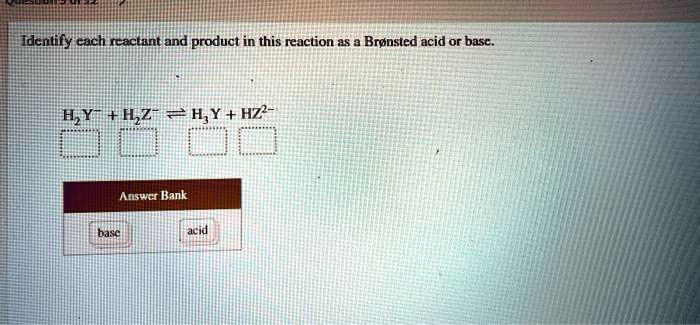
SOLVED: Identify each reactant and product in this reaction as a Brónsted acid or base? H2Y- + H2Z- <—> H3Y + HZ2- Identily cach ractant and product in this reaction as Bronsted

Highly Selective Surface Lewis Acid−Base Reaction: Trimethylamine on Si(100)c(4×2) | The Journal of Physical Chemistry B
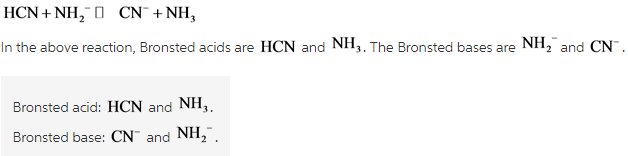
Label each reactant and product in this reaction as a Bronsted acid or base - Home Work Help - Learn CBSE Forum

acid base - How to predict the color of a pH indicator using Le Chatelier's principle - Chemistry Stack Exchange

SOLVED: 30.At what pH would you expect the aminoacid prolinePto be fully protonated?pK=1.952,pK2=10.640 A.1.952 B.1.750 C.2.500 D.10.640 E.7.000 31.Given pKvalues of citric acid2-hydroxypropane-1,2,3-tricarboxylic acid,pK=3.128,pK=4.761,and pK3= 6.396 ...