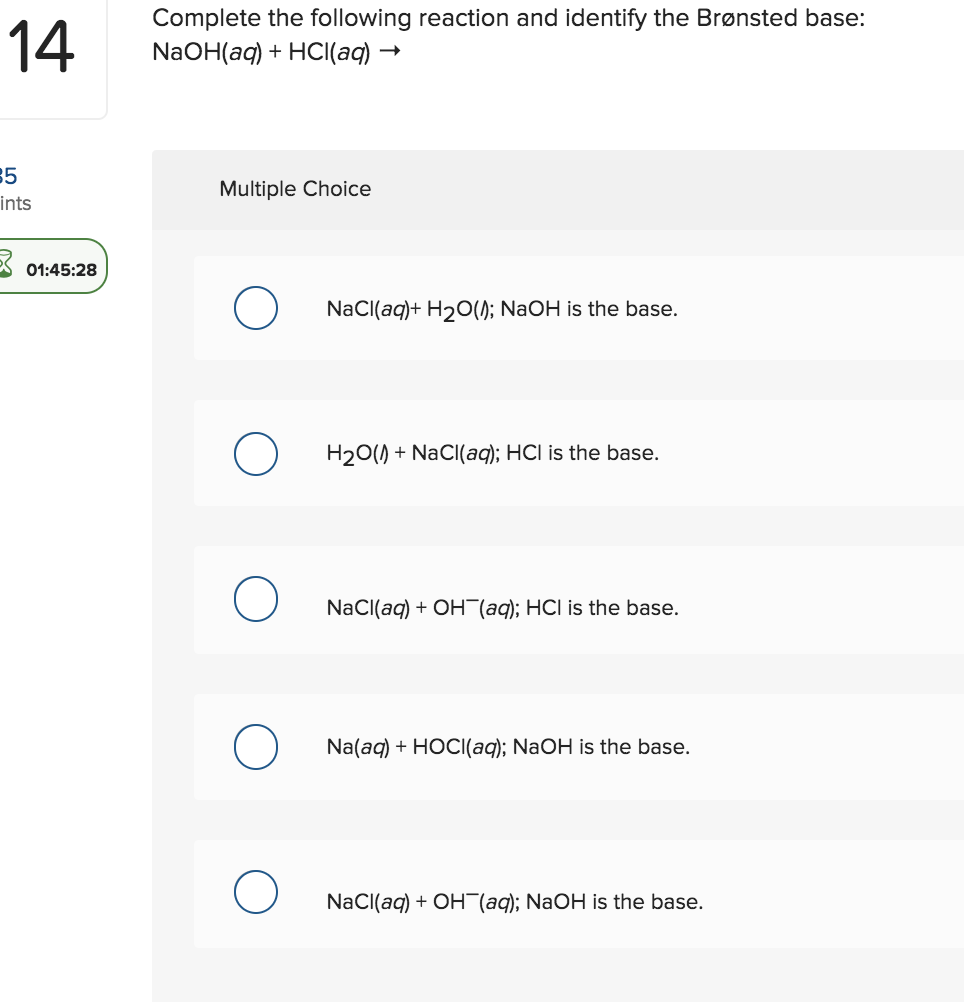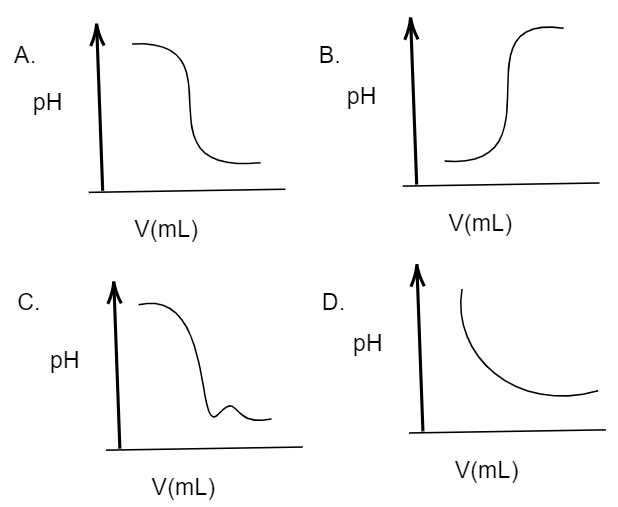
In an acid-base titration, $0.1M$ $HCl$ solution was added to the $NaOH$ solution of unknown strength. Which of the following correctly shows the change of pH of the titration mixture in this
Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stock Illustration - Download Image Now - iStock

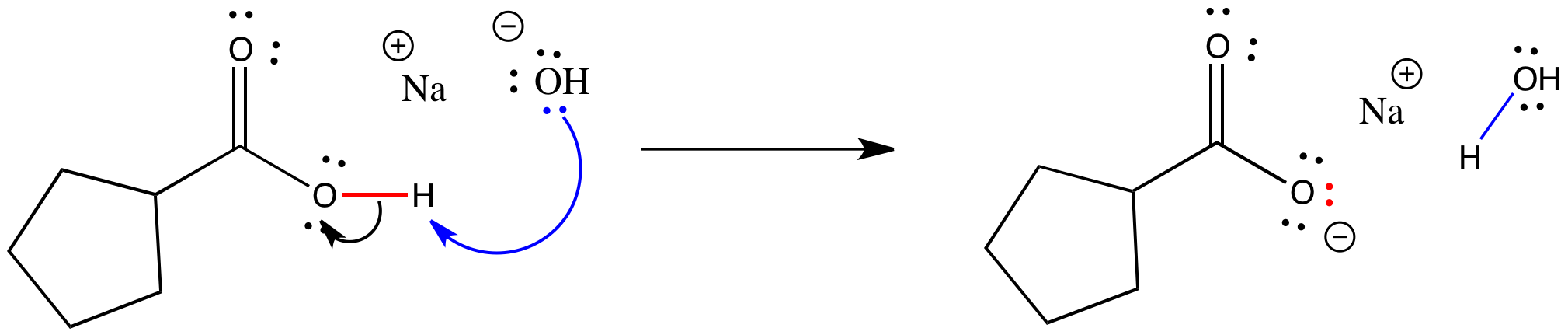
Show how the given species can act as Lewis bases in their reactions with HCl. CH_3CH_2OH, (CH_3)_2NH, (CH_3)_3P | Homework.Study.com

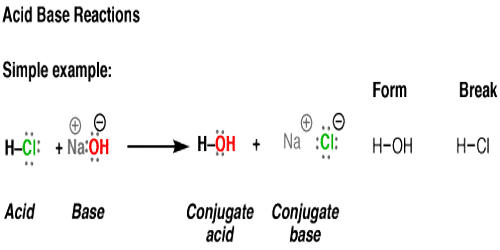
Acid – base reaction. chemical reaction neutralization the acid and base properties, producing a salt and water. used to determine pH. Bronsted – Lowry theory. molecules of HCl, NaOH, H2O, and NaCl,

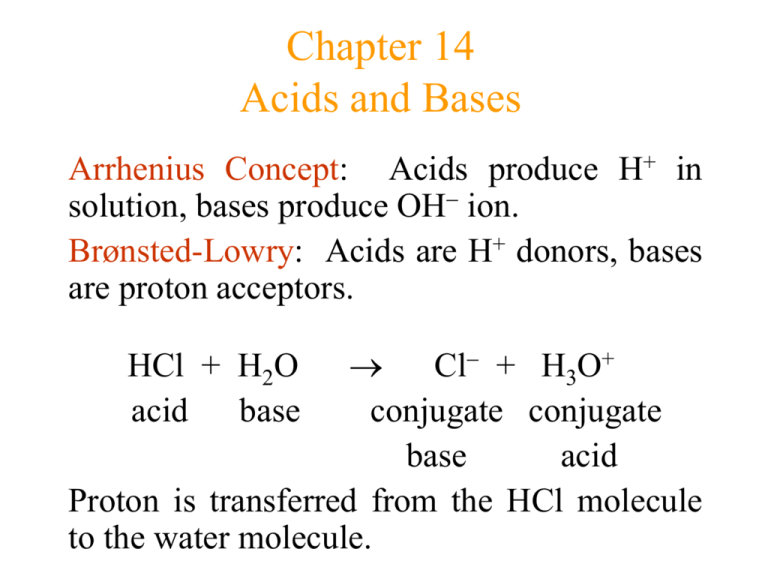
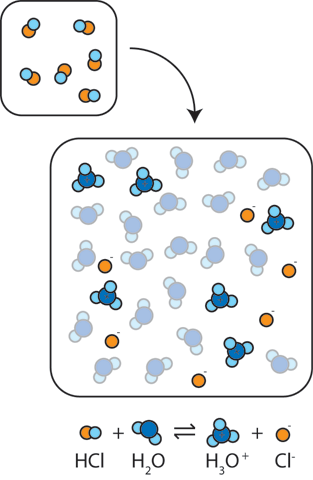
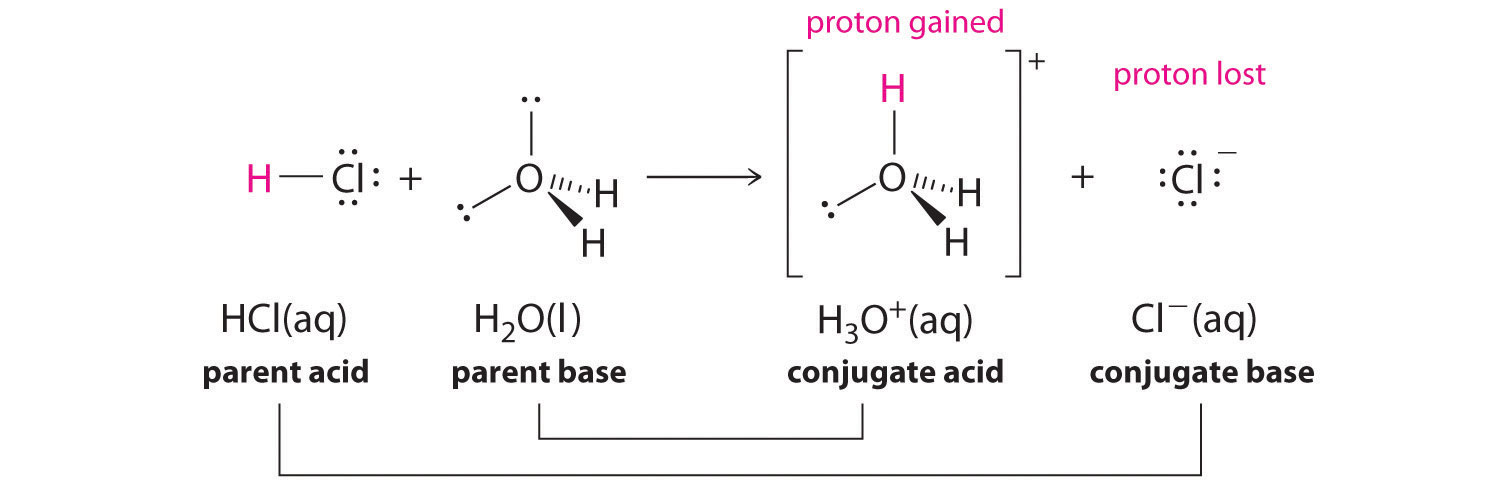
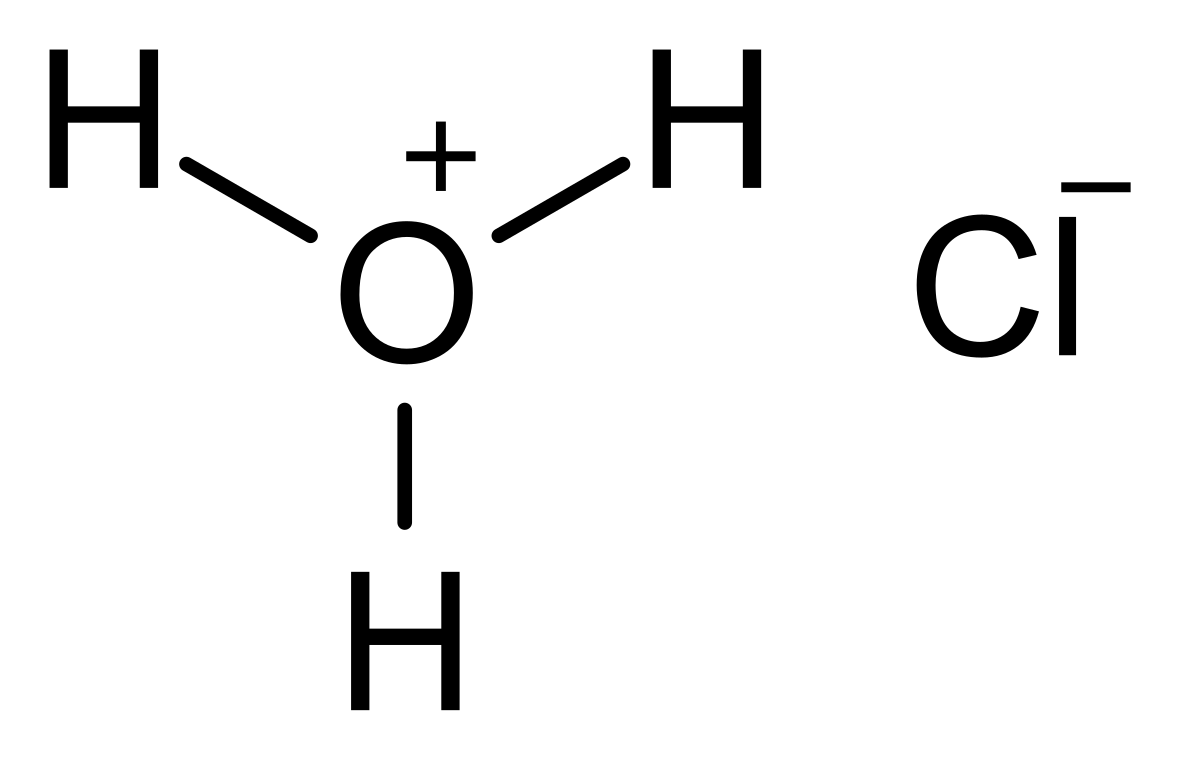
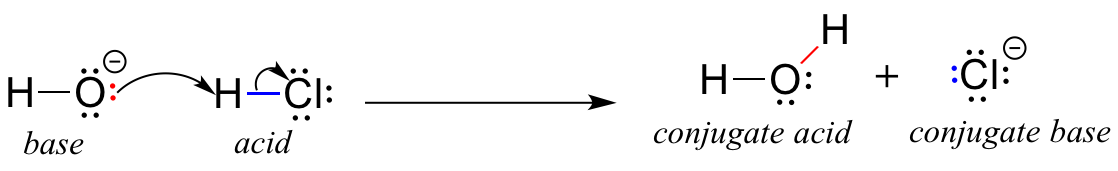
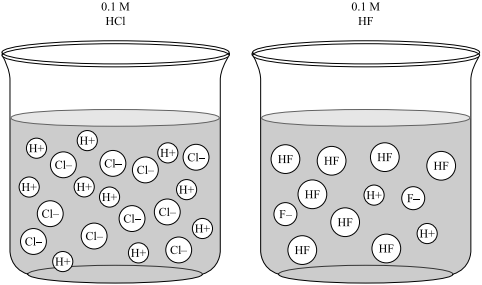
The ionization of hydrochloric acid in water is given below: HCl(aq) + H2O(l) H3O^+(aq) + Cl^-(aq) Lable two conjugate acid - base pairs in this ionization.